Characterisation of Disintegration of Pharmaceutical Solid Dosage Forms
Even though the majority of solid dosage forms on the market and in development are immediate release tablets, surprisingly little is known about the disintegration process. In traditional disintegration testing it is difficult to establish any detailed insights into the mechanism of tablet disintegration as the test is merely designed to indicate the time it takes for a tablet or capsule to disintegrate completely, and this is defined as the state "in which any residue of the unit [...] is a soft mass having no palpably firm core". Based on the results of the disintegration test it is judged whether or not the dosage form meets the specification required by the respective pharmacopoeia e.g. for immediate release formulations. Apart from establishing the conformity with such official guidelines the disintegration test yields little additional information and is not very useful to guide rational formulation design.
The development of immediate release formulations is still dominated by a largely empirical approach. One reason for this situation could be due to the lack of sufficiently fast and accurate measurement techniques to quantify the changes in microstructure that occur during the interaction of the dosage form and the dissolution medium. Here we introduce a fundamentally new approach based on terahertz pulsed imaging (TPI) to investigate the penetration of dissolution medium into the porous matrix of immediate release formulations. Using this technique it is possible to measure the liquid transport into the dosage form in detail and extract quantitative data that can be used to design mathematical models forming the basis for rational formulation design for immediate release tablets.
Fast and Non-destructive Imaging of Disintegration
We recently developed a flow cell that can be used for measuring the one-dimensional liquid ingress during disintegration of immediate release tablets by TPI. The terahertz measurement is a non-contact and non-invasive method that is performed at the centre of flat faced tablets with a time resolution of less than 1 s per sampling point [1,2]. Using this method it is possible to simultaneously measure the amount of overall swelling of the tablet as well as the position of the liquid front within the tablet. The method exploits the fact that terahertz radiation can penetrate deep into pharmaceutical powder compacts due to the transparency of excipients at terahertz frequencies as well as the fact that there is a significant difference in the refractive index between dry polymer and dissolution medium.
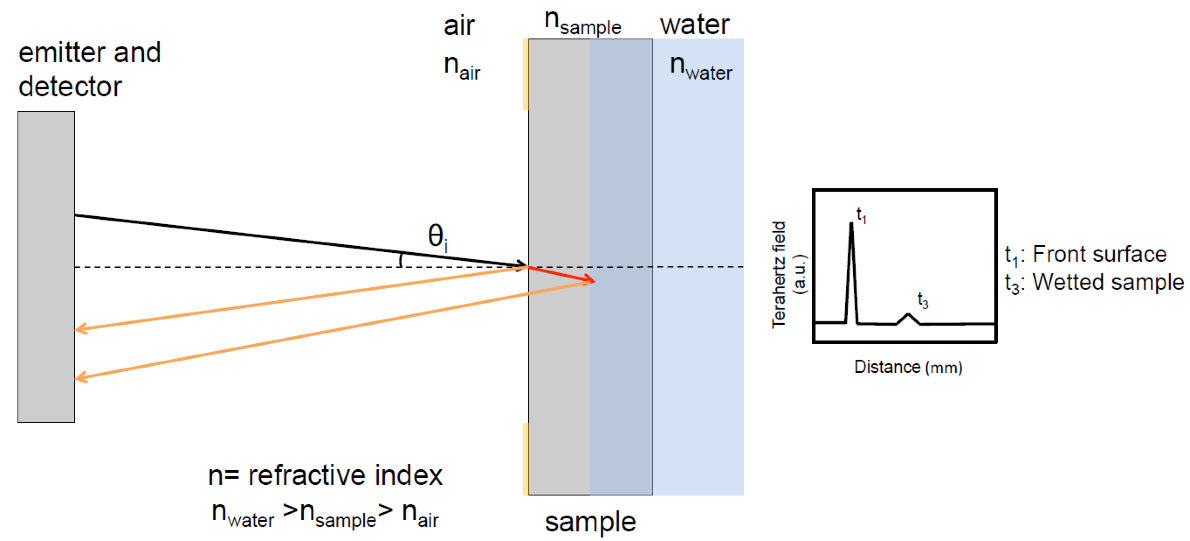
Characterisation of Microstructural Changes during Disintegration
We demonstrated how TPI can be used to simultaneously characterise the liquid ingress, the swelling of the tablet, and cracks (formed within the powder compact during hydration). Combining the information from the terahertz reflection measurements about liquid penetration, swelling and the formation of cracks provides fundamental information about the microstructural behaviour of complex powder compacts.
Our work indicated that subtle differences in formulation have profound effects on the disintegration. A change in crosscarmellose sodium concentration from 2 to 5 wt% has a dramatic effect on the disintegration kinetics, particularly at a water temperature of 20°C. Here the disintegration time of the tablet is one order of magnitude faster at the higher concentration of superdisintegrant. Our work also highlights the enormous effect of the temperature of the dissolution medium on how rapidly a tablet disintegrates: by changing the temperature from 37°C to 20°C the disintegration time reduced from 25 to 5 seconds in a tablet containing 5% croscarmellose sodium (see Figure below).
![Disintegration characteristics of tablets made from MCC and superdisintegrant at different concentrations and water temperature (modified from [1]).](https://thz.ceb.cam.ac.uk/files/styles/leading/public/media/Dis_results.png?itok=a_fdWh-7)
The new method is universally applicable to a wide range of formulations for dosage forms with disintegration times from seconds to hours. The quantitative study of the hydration process and relating the extracted information to material properties and process configurations is of paramount importance for the development of new products and for assuring a high and consistent quality. Clearly, advances in understanding mass transport mechanisms will also be of central importance for many other application areas outside the pharmaceutical industry
Free Download
For the full details please click on the link below to the papers:
[1] S. Yassin, D. Goodwin, A. Anderson, J. Sibik, D.I. Wilson, L.F. Gladden and J.A. Zeitler, The disintegration process in microcrystalline cellulose based tablets I.: influence of temperature, porosity and superdisintegrants, Journal of Pharmaceutical Sciences, doi:10.1002/jps.24544 (2015).
[2] P. Bawuah, P. Silfsten, T. Ervasti, J. Ketolainen, J.A. Zeitler, K.-E. Peiponen, Non-contact weight measurement of flat-faced pharmaceutical tablets using terahertz transmission pulse delay measurements, International Journal of Pharmaceutics, doi:10.1016/j.ijpharm.2014.09.027, 476, 16-22 (2014). Free download on Researchgate
